Regulatory Impact Assessment (RIA)
for Medical Devices
Smarter, Faster Regulatory Impact Assessment in Days (not Weeks)
In today’s fast-changing regulatory environment, medical device companies must keep pace with frequent updates from bodies like the EU’s Notified Bodies, FDA, MHRA, and other global health authorities. The introduction of MDR, IVDR, and similar frameworks has only increased the complexity and compliance burden.
DDi’s AI-powered Regulatory Impact Assessment (RIA) solution from Visu, helps medical device manufacturers transform their compliance process from reactive and manual to proactive, scalable, and intelligent.
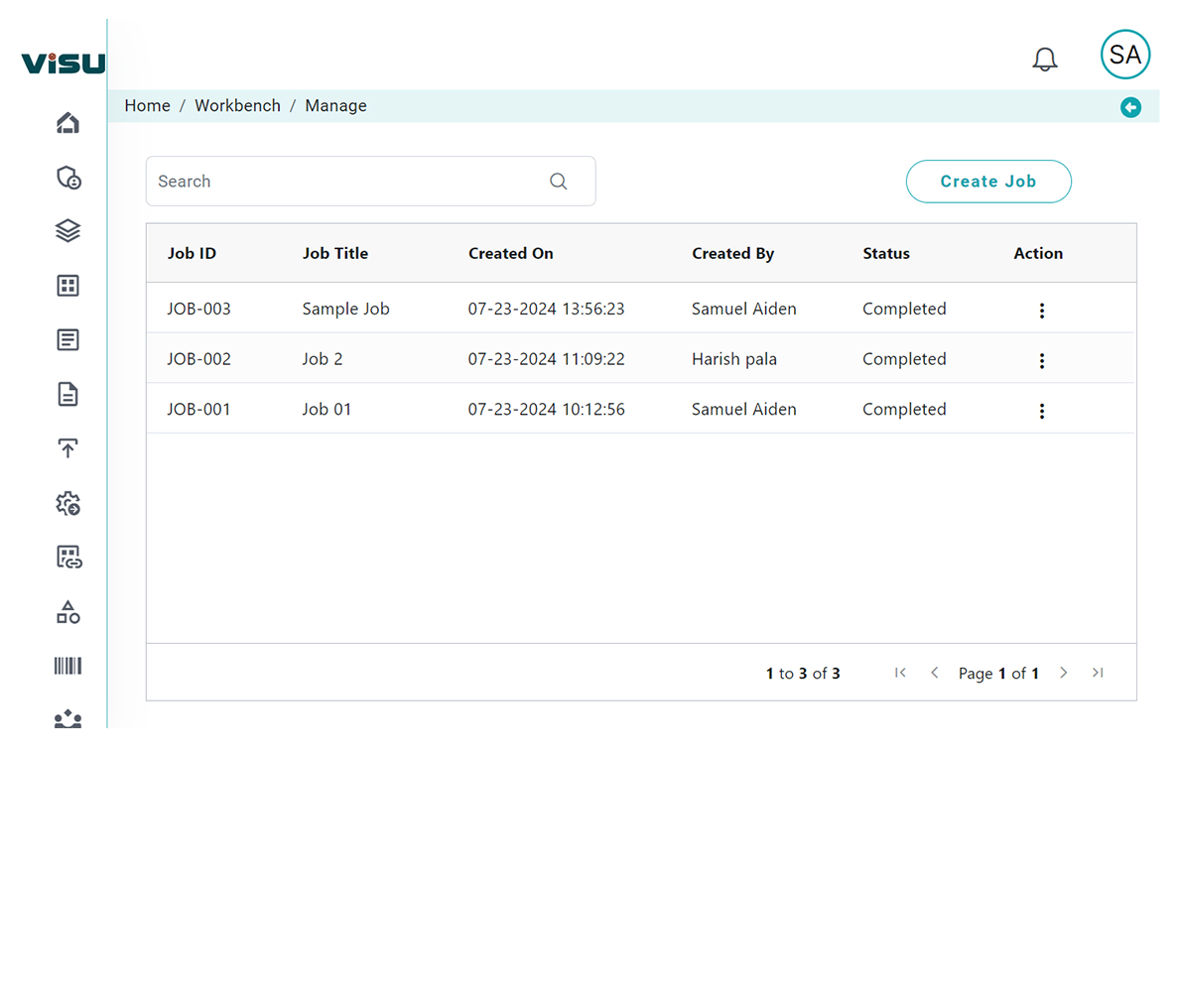
The Challenge: Fragmented and Manual RIA
Regulatory changes can lead to a ripple effect across labeling, design history files, clinical evaluation reports, IFUs, and technical documentation. However, most organizations still depend on:
- Spreadsheets and static reports for change tracking
- Manual interpretation of regulatory texts
- Isolated data silos across regulatory, engineering, and QA teams
- Reactive planning after updates are already in effect
This traditional approach makes it difficult to identify relevant changes quickly, act decisively, or ensure full traceability during audits.
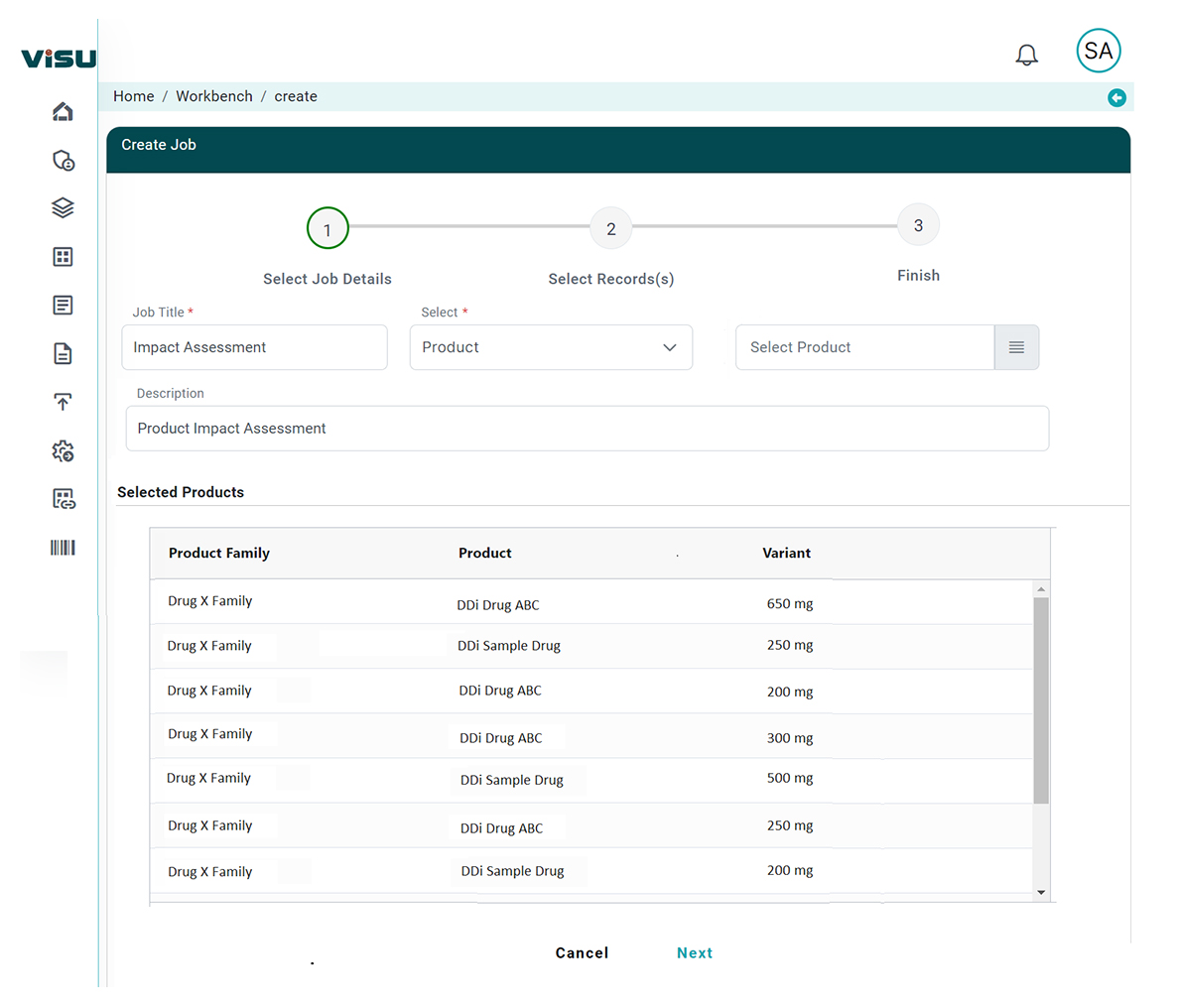
AI-Driven RIA Solution, powered by Visu
By embedding advanced AI into the core of our platform, Visu automates and augments every stage of the RIA process:
Automated Regulatory Monitoring
AI bots scan global health authority sites – including MDR/IVDR notices and FDA announcements – using NLP to identify relevant updates in near real-time.
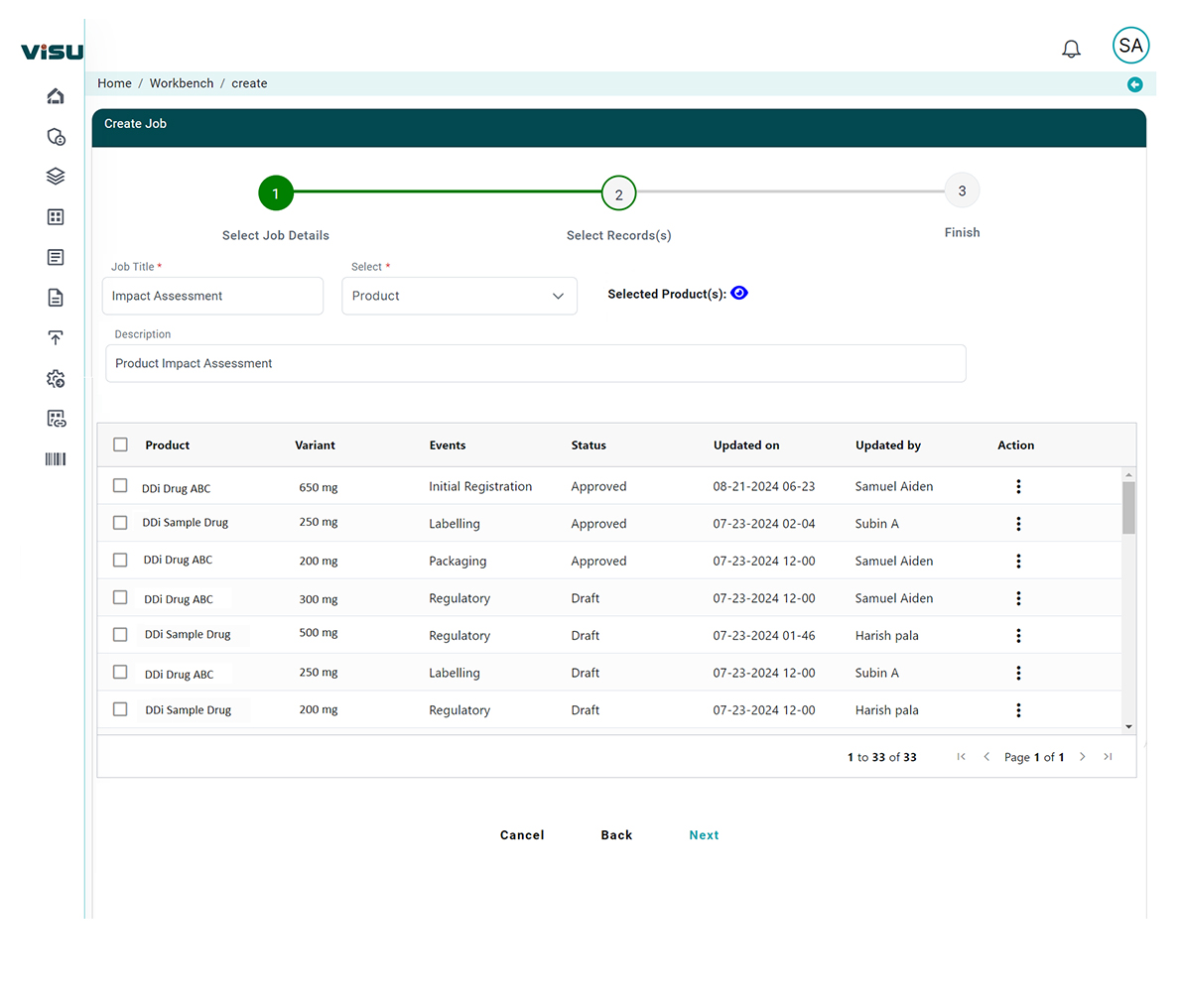
Contextual Impact Mapping
Our AI models match changes directly to device categories, design documentation, and submission data – eliminating guesswork.
Risk-Based Prioritization
AI evaluates the scope and severity of changes, enabling you to triage responses based on risk level and business impact.
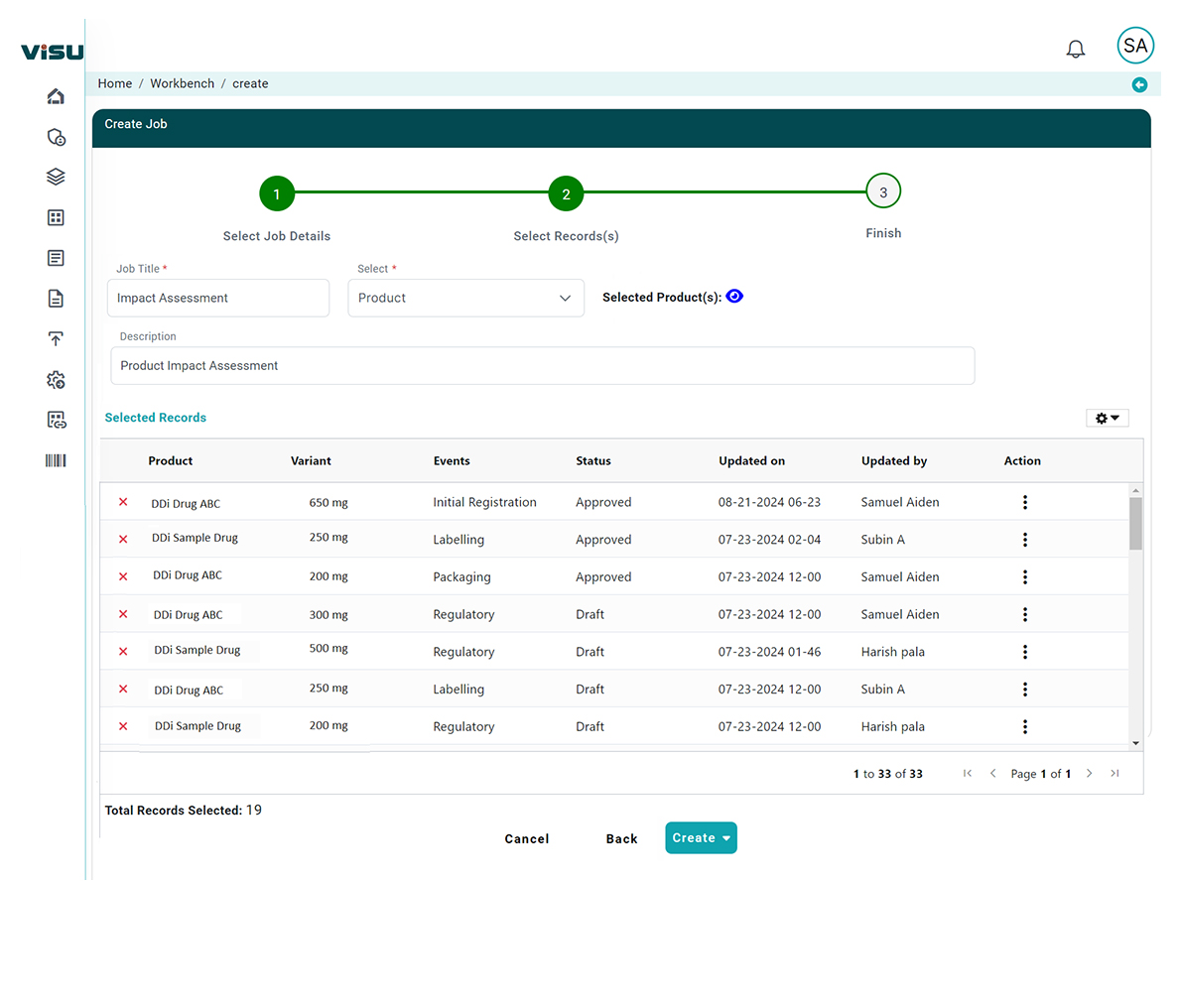
Automated Alerts & Cross-Functional Collaboration
Trigger workflows and alerts across RA, engineering, labeling, and quality teams with auto-assigned tasks and timelines.
Benefits of AI-Enabled RIA for MedTech
- Faster Turnaround: Reduce manual analysis time by up to 80%
- Greater Accuracy: AI minimizes human error in mapping and interpretation
- Actionable Insights: Prioritize based on real business and compliance risk
- Global Scalability: Manage compliance across diverse regions and device lines without increasing headcount
- Audit-Ready Documentation: Complete traceability and version control for every decision and action
Ready to Simplify and Strengthen Your Regulatory Strategy?
Talk to our solution team to learn how AI-powered Regulatory Impact Assessment can keep your assessment process faster and effective.
Book a demo or consultation today.
Let's talk about how DDi can help you


