Regulatory Impact Assessment (RIA)
for Pharma & Biotech
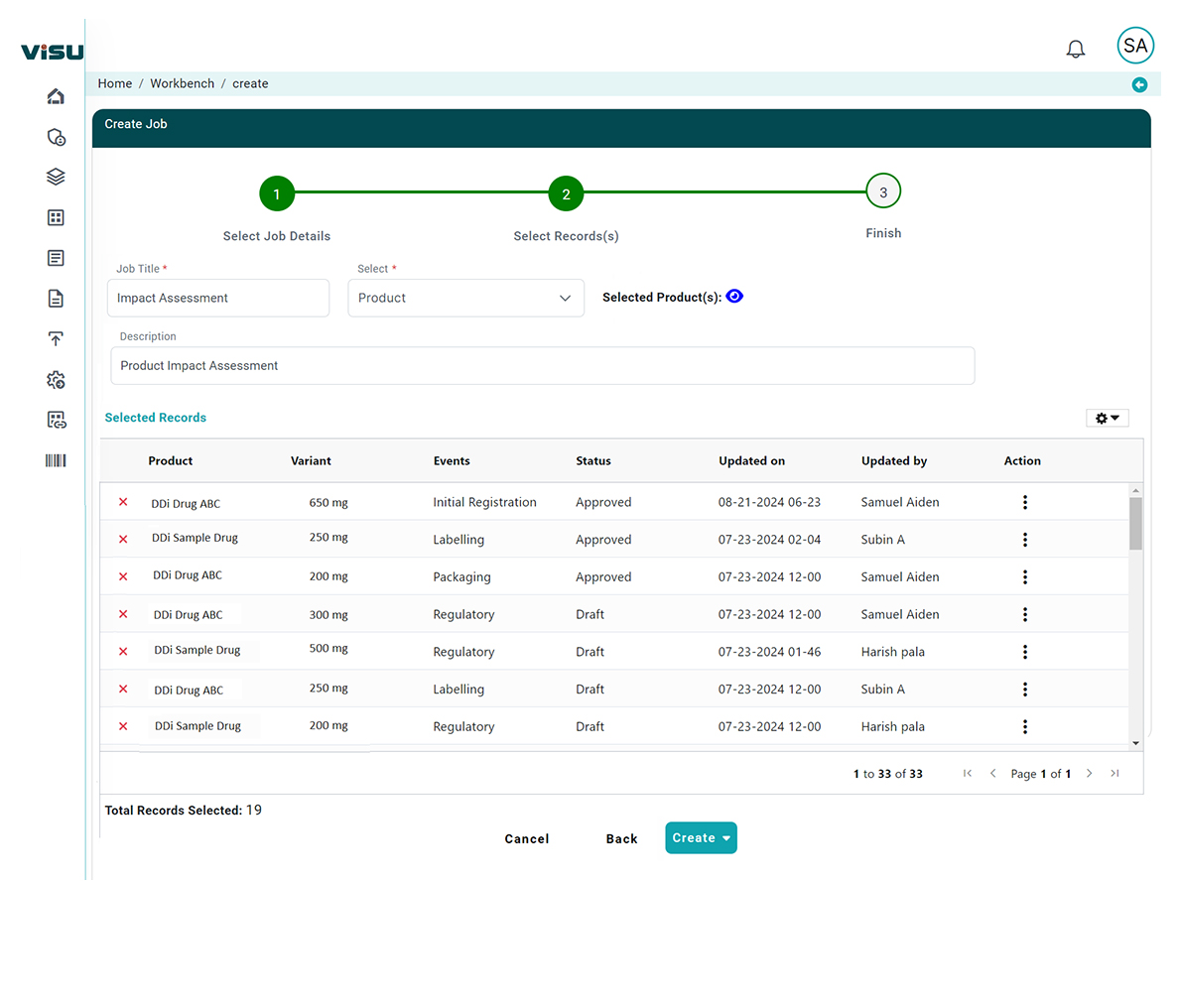
AI-Powered Regulatory Impact Assessment in Days (not Weeks)
As pharmaceutical and biotech companies operate in an increasingly dynamic global regulatory environment, the ability to quickly and accurately assess regulatory impact is mission-critical. With evolving guidelines from the FDA, EMA, MHRA, and other health authorities, even minor changes in regulations can cascade into major revisions in labeling, safety submissions, or clinical documentation.
DDi’s Regulatory Impact Assessment (RIA) solution, powered by Visu, helps pharma and biotech organizations seamlessly navigate this complexity with an AI-powered approach that enhances speed, accuracy, and strategic agility.
Traditional RIA Challenges in the Pharma Industry
Most companies still rely on outdated, manual methods for conducting RIAs – spreadsheets, emails, and siloed document repositories. These present significant limitations:
- No Real-Time Updates – Manual tracking often leads to missed or late responses to regulatory changes.
- Siloed Teams – Disconnected RA, safety, clinical, and labeling teams result in poor cross-functional coordination.
- Inconsistent Mapping – Matching regulatory changes to the correct product, region, or dossier is time-consuming and error-prone.
- Reactive Posture – Traditional RIA starts after changes are published – too late for many time-critical updates.
The result? Risk of non-compliance, market delays, or even costly recalls.
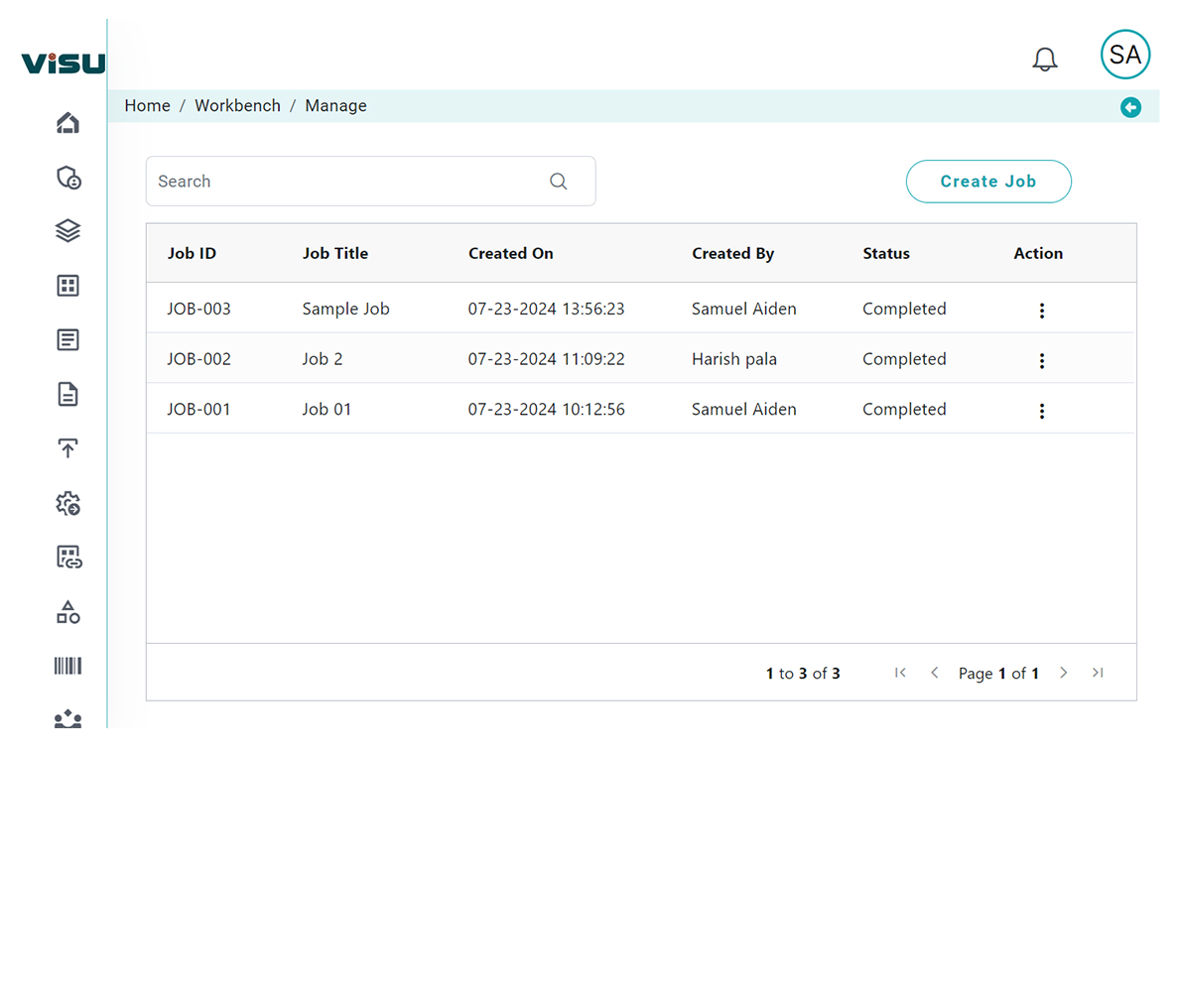
How we Transform RIA Using Artificial Intelligence
Leveraging AI and machine learning, DDi’s Visu introduces intelligence, automation, and scalability into the regulatory impact assessment process:
Automated Regulatory Monitoring
Our AI engines continuously scan global regulatory databases, including FDA, EMA, and ICH sources. NLP (Natural Language Processing) interprets and classifies regulatory changes in real-time.
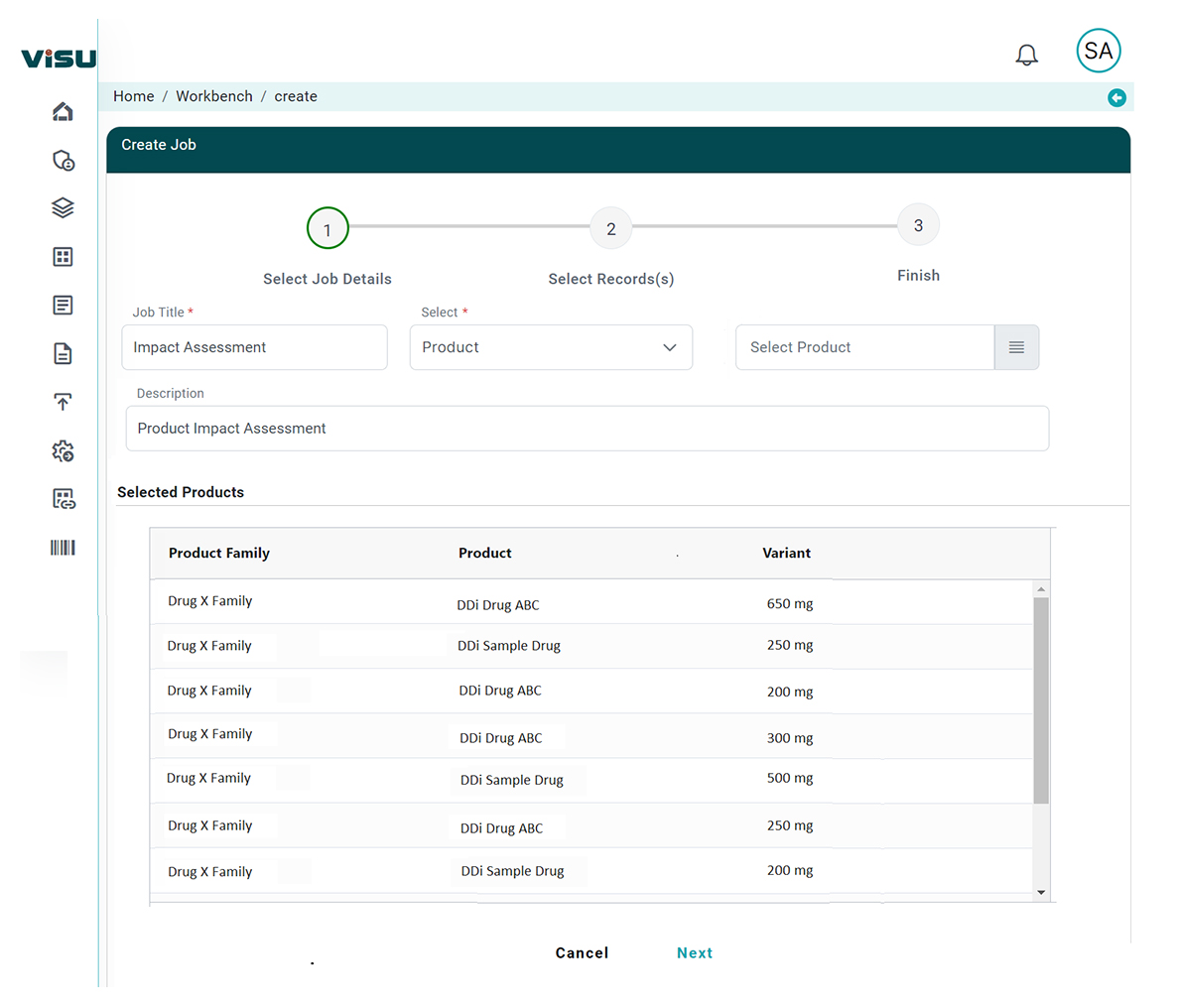
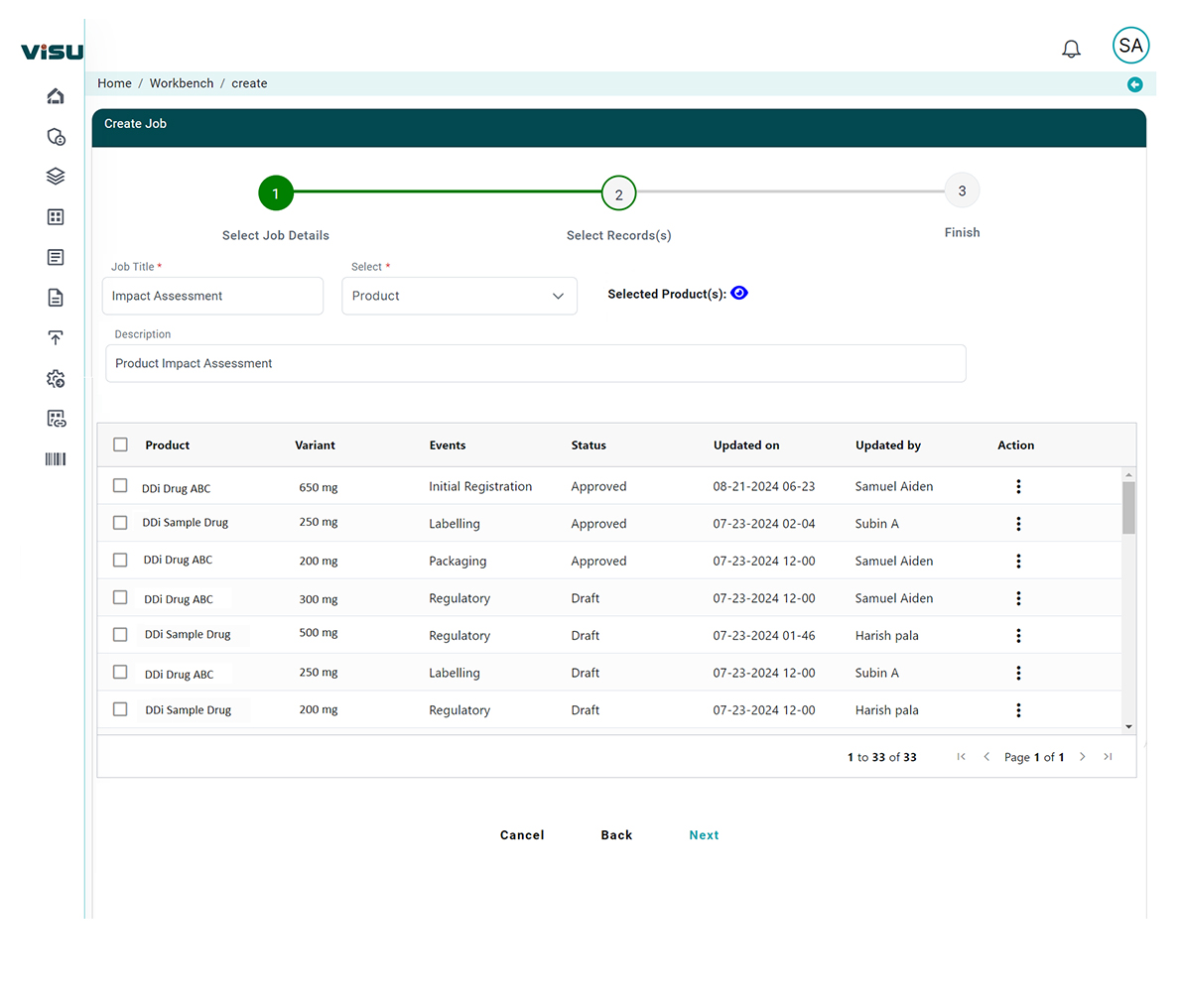
Intelligent Impact Mapping
AI links new regulations directly to affected product dossiers, submission components, safety documents, and labeling – reducing hours of manual work.
Risk-Based Prioritization
Visu automatically assesses the severity and business risk of each regulatory update, helping teams focus on what matters most.
Cross-Functional Alerting & Collaboration
Built-in workflows notify stakeholders across departments – regulatory, clinical, safety, and labeling – enabling coordinated, timely responses.
Key Benefits for Pharma Regulatory Teams
- Accelerated Turnaround: Reduce impact assessment time from weeks to hours
- Improved Accuracy: Eliminate manual errors through AI-powered document and metadata mapping
- Global Scalability: Efficiently manage hundreds of products across multiple markets
- Proactive Compliance: Stay ahead of changes before deadlines approach
- Insight-Driven Decision-Making: Prioritize based on risk and business value with predictive analytics
Technologies Powering Visu’s AI-Driven RIA
- Natural Language Processing (NLP): Reads and classifies regulatory texts
- Machine Learning (ML): Continuously improves with historical data and decisions
- Predictive Analytics: Forecasts downstream impact and potential risks
- Agentic-AI Models: Multiple agents simulate expert reasoning and actions
Take the Lead in Regulatory Excellence
The cost of outdated, manual RIA is high. With Visu, your RA team can transform impact assessments into a strategic asset – responding faster, smarter, and more confidently than ever before.
Talk to us today to see how AI-driven RIA can revolutionize your compliance strategy.
Let's talk about how DDi can help you