Automated Publishing &
Submission management solutions
for Pharma & Biotech
In the highly regulated world of life sciences, document publishing is a critical yet time-intensive step in regulatory submissions. Manual publishing processes can lead to errors, delays, and higher costs, making it difficult for teams to stay compliant and efficient.
DDi’s Automated Publishing Solutions eliminate these challenges by intelligently automating every step of the publishing process, from document preparation to final validation, ensuring FDA-compliant and submission-ready outputs in minutes.
Time & Cost Savings: Our automated publishing software empowers pharma and biotech companies to reduce manual efforts, eliminate rework, accelerate submission cycles, and cut publishing costs by over 70%, guaranteed

Why Choose DDi’s Publishing Automation Platform?
Unlike traditional publishing tools that rely on manual intervention, DDi’s publishing automation system leverages intelligent rule-based processing, integrations, and AI-assisted workflows. Whether your files are in MS Word or PDF, our solution delivers submission-ready outputs aligned with global regulatory standards, seamlessly integrating with your existing eCTD, RIM, and EDMS environments.
Key Benefits
- Drastically Reduce Manual Work – Automate repetitive publishing steps such as formatting, hyperlinking, TOC creation, and validation.
- Faster Time to Submission – Generate compliant files in minutes, not days.
- Save 70% or More on Publishing Costs – Cut cycle time and operational expenses while improving quality.
- Ensure FDA and Global Compliance – Preconfigured with out-of-the-box rules for FDA, EU, and global health authorities.
- Seamless Integration –Connect effortlessly with your current RIM, EDMS, Documentum, SharePoint, Box, and other systems.
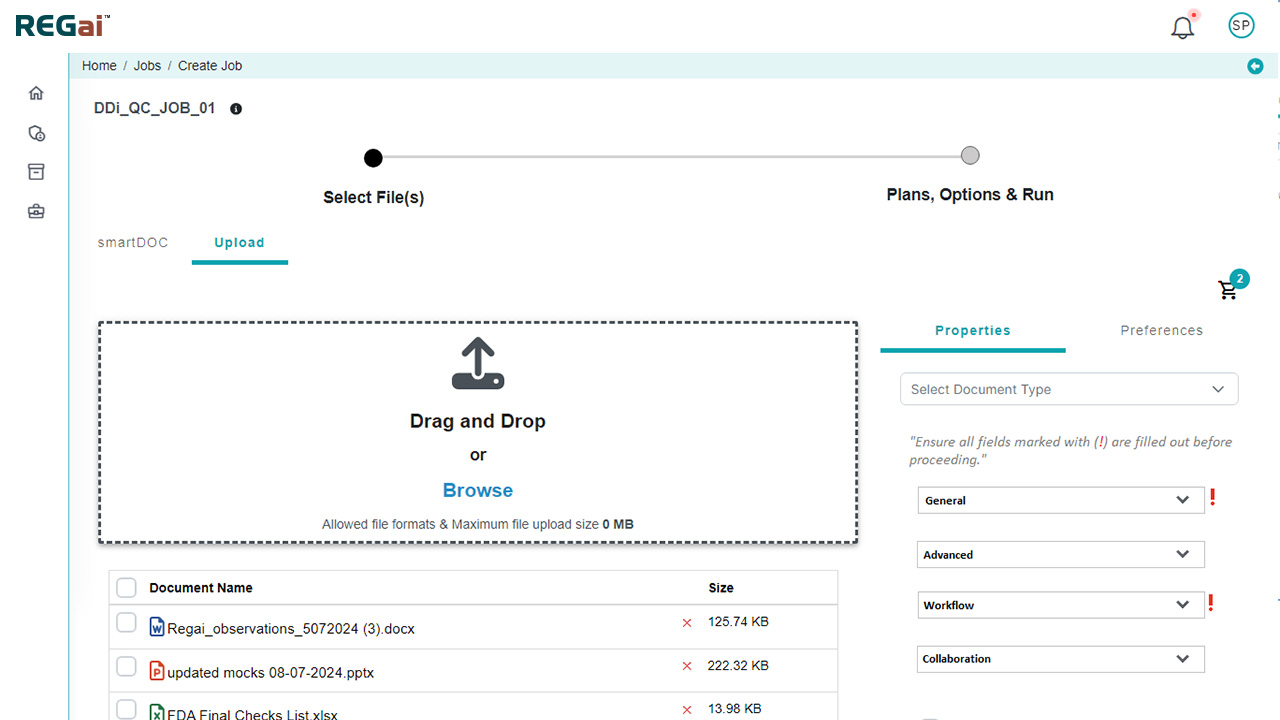
Pre-Publishing Automation: Intelligent Document Processing
Our automated publishing software includes a powerful 450-rule library designed to standardize and enhance document preparation.Built-in Publishing Rules
Predefined templates and configurations cover:
- Tables, fonts, TOC, headers/footers, bookmarks, and hyperlinks
- File naming and metadata alignment
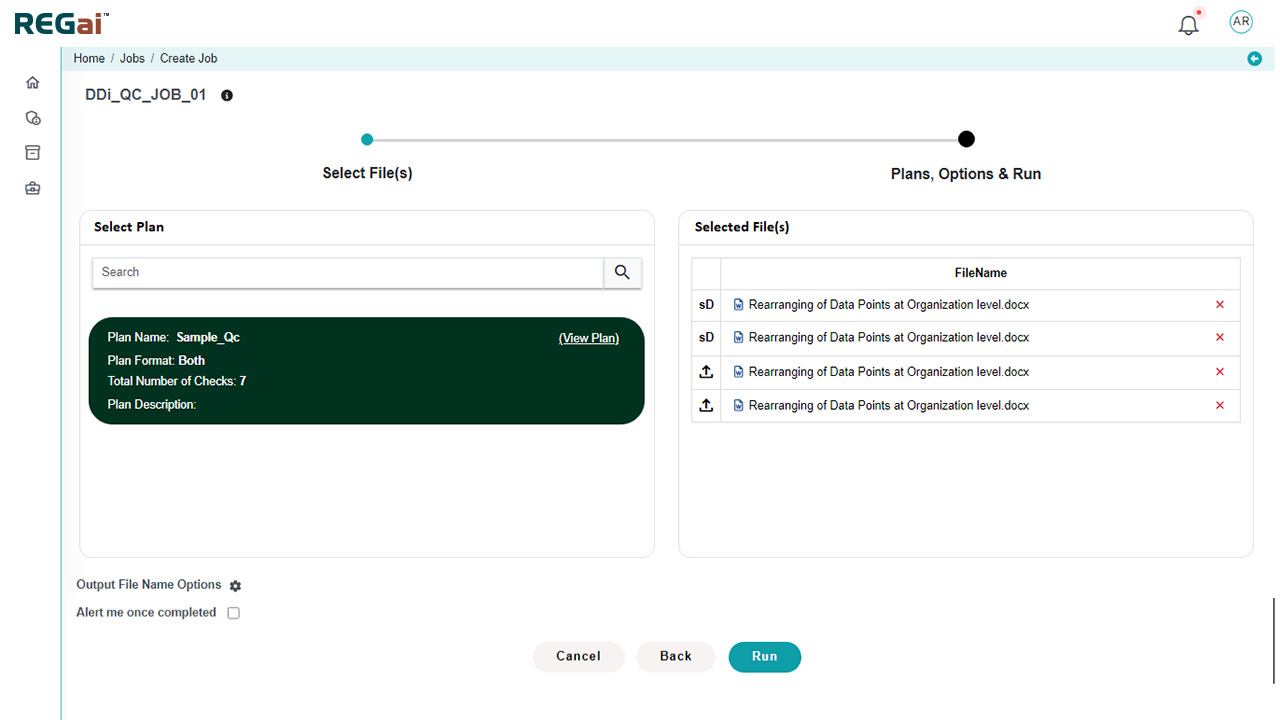
- Standard and custom publishing plans for global agencies
Multiple Ways to Use
Choose the method that best fits your workflow:
- Word Plugins for direct document publishing
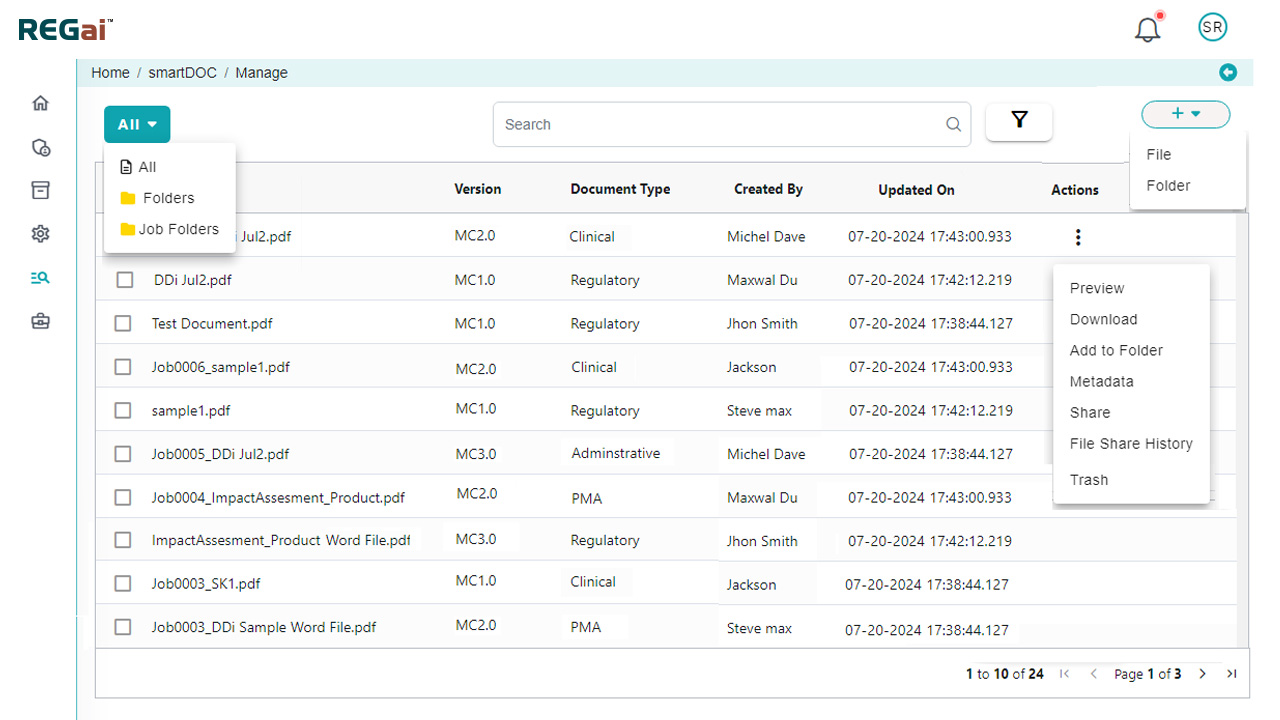
- REGai Tool for quick uploads from desktop or EDMS
- Chatbot Interface for intuitive, faster uploads/downloads
- Batch Processing for scheduled publishing (hourly, daily, or custom intervals)

Post-Publishing Automation: Compliance and Validation Made Simple:
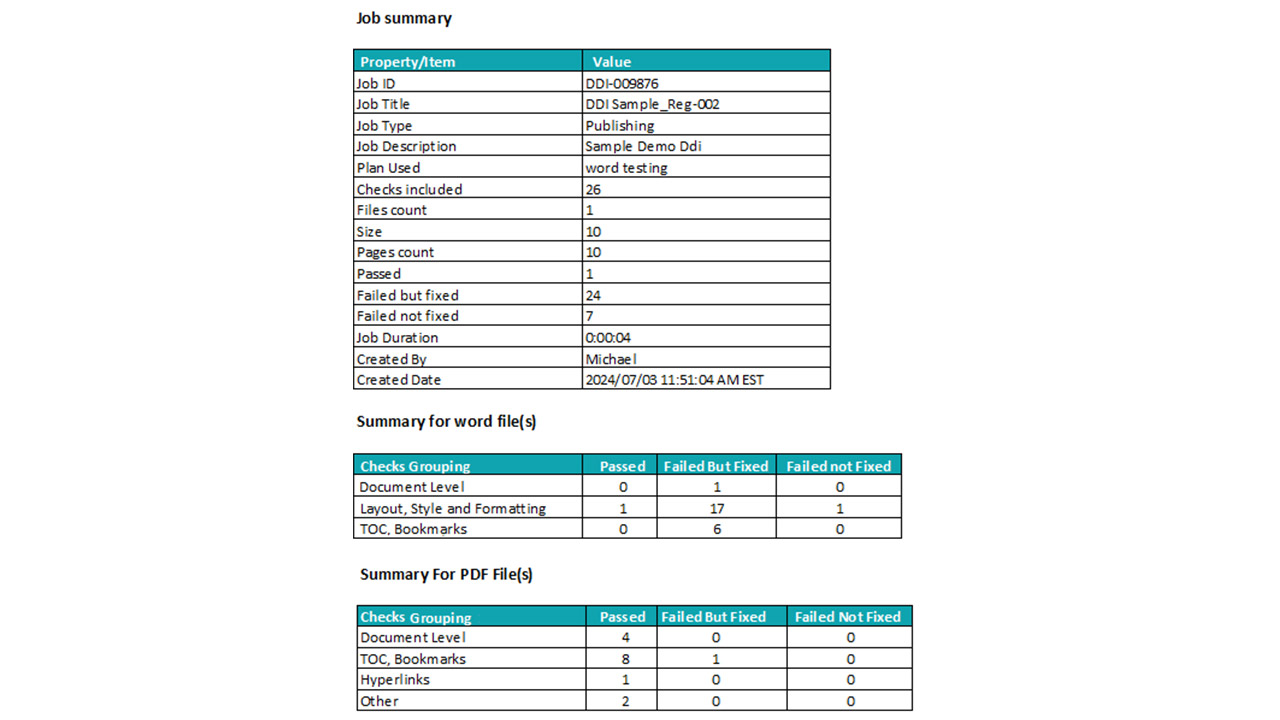
Once publishing is complete, our publishing automation platform continues to ensure quality and compliance through built-in validation features.
Automated Validation & Checks
- Automated dossier validation against custom or standard checklists
- Verification of dossier structure, titles, and data content
- Inter-document hyperlink verification and auto-updates
- File name and leaf title alignment with internal standards
- Selective testing of validation paths to ensure accuracy
With these intelligent automation capabilities, your publishing team can eliminate human error and guarantee submission accuracy across multiple regions.
Seamless System IntegrationDDi’s automated publishing solutions come with pre-built connectors for easy integration with your existing enterprise systems:
- eCTD systems
- Regulatory Information Management (RIM) platforms
- Electronic Document Management Systems (EDMS)
- ERP, SharePoint, Documentum, Box, and other third-party applications
This ensures smooth data flow, consistent document management, and minimal disruption to your current infrastructure.
Accelerate Compliance and Reduce Publishing Costs
With DDi’s publishing software FDA-ready framework, companies can ensure faster, compliant, and high-quality dossier creation for global submissions.
By automating key steps across pre-publishing, publishing, and post-publishing phases, DDi helps regulatory teams achieve:
- 70%+ time and cost savings
- 90% fewer publishing errors
- 100% audit-ready compliance
Our automated publishing software transforms publishing into a strategic advantage, enabling faster approvals, smoother audits, and enhanced operational efficiency.
Experience the Future of Regulatory Publishing
DDi’s automated publishing solutions are trusted by leading pharma and biotech organizations to modernize their regulatory publishing processes. Whether you need complete publishing automation or seamless integration with your existing eCTD systems, we have the expertise and technology to make it happen.
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you


